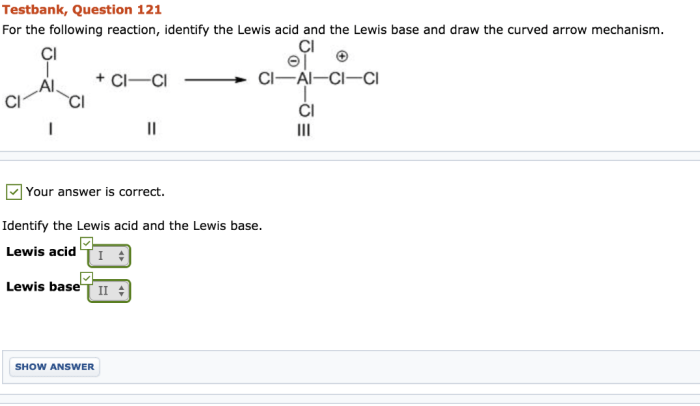
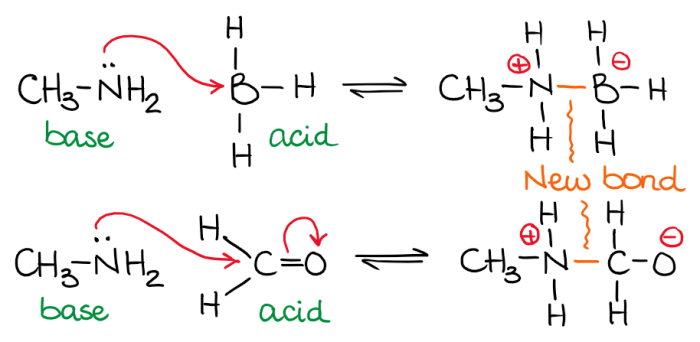
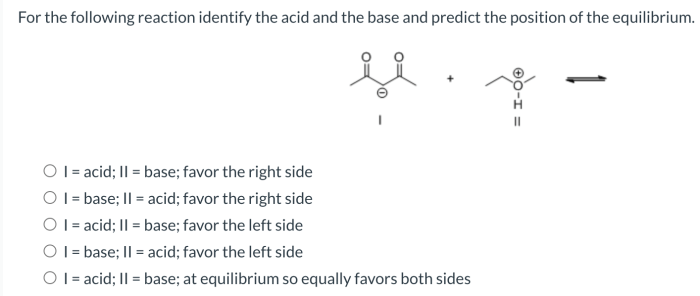
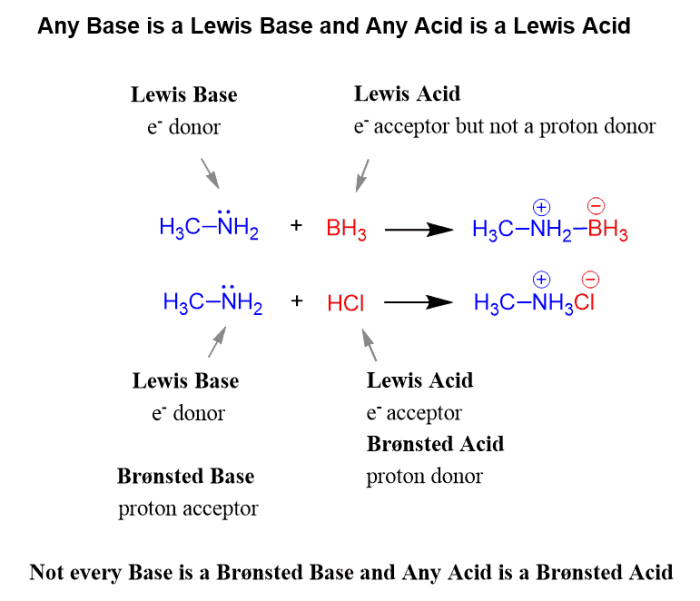For the following reaction identify the lewis base – In chemistry, the concept of Lewis bases plays a crucial role in understanding the behavior of molecules and their interactions. Lewis bases are electron-pair donors, and their identification in chemical reactions is essential for comprehending the reaction mechanism and predicting the products formed.
This article delves into the identification of Lewis bases in a given reaction, exploring the characteristics of Lewis bases, analyzing the reactants and products involved, and providing evidence from the reaction mechanism to support the identification.
Lewis Base Identification and Reaction Analysis: For The Following Reaction Identify The Lewis Base
A Lewis base is a substance that can donate an electron pair to form a covalent bond. Lewis bases are typically electron-rich species with lone pairs of electrons. In a chemical reaction, a Lewis base reacts with a Lewis acid, which is a substance that can accept an electron pair.
Reaction Analysis
Consider the following reaction:
“`NH 3+ HCl → NH 4Cl“`
In this reaction, NH 3is the Lewis base and HCl is the Lewis acid. NH 3donates its lone pair of electrons to HCl, forming a covalent bond between the nitrogen atom in NH 3and the hydrogen atom in HCl.
Lewis Base Identification in the Reaction, For the following reaction identify the lewis base
To identify the Lewis base in a reaction, look for the substance that donates an electron pair. In the reaction above, NH 3is the Lewis base because it donates its lone pair of electrons to HCl. This is supported by the fact that NH 3has a lone pair of electrons, while HCl does not.
Comparison of Lewis Base Properties
Lewis bases vary in their strength, which is determined by the availability of their lone pairs of electrons. Some common Lewis bases include:
- NH 3
- H 2O
- OH –
- CN –
These Lewis bases have varying strengths, with CN –being the strongest and NH 3being the weakest.
Q&A
What is a Lewis base?
A Lewis base is a chemical species that can donate an electron pair to form a covalent bond.
How do you identify a Lewis base in a reaction?
To identify a Lewis base in a reaction, look for the species that donates an electron pair to form a bond with another species.
What are some common examples of Lewis bases?
Common examples of Lewis bases include hydroxide ions (OH-), ammonia (NH3), and water (H2O).