Which sequence of reagents will best accomplish this transformation? This question sets the stage for an engaging exploration into the realm of organic chemistry, where the judicious selection of reagents is paramount to achieving desired molecular transformations. Understanding the interplay between functional groups, reaction mechanisms, reagent compatibility, and reaction optimization empowers chemists to navigate the intricate tapestry of chemical synthesis with precision and efficiency.
Delving into the intricacies of functional group identification, we uncover the significance of spectroscopic techniques in deciphering the molecular architecture of organic compounds. This knowledge serves as a cornerstone for selecting appropriate reagents that can selectively target specific functional groups, paving the way for targeted chemical transformations.
Identifying Functional Groups
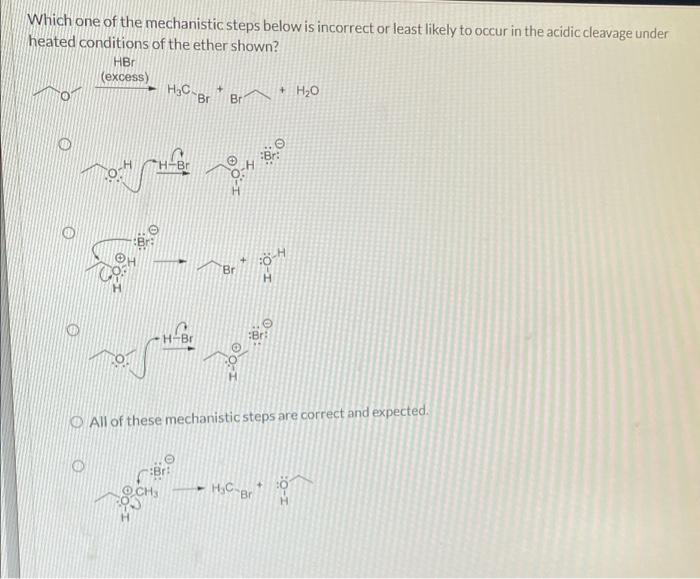
Identifying functional groups is crucial for selecting appropriate reagents. Functional groups are specific groups of atoms within a molecule that determine its chemical properties. By identifying the functional groups present in a molecule, one can predict its reactivity and choose reagents that will selectively target those groups.
Common functional groups include alcohols, aldehydes, ketones, carboxylic acids, and amines. Each functional group has its own characteristic reactivity and requires specific reagents for its transformation.
Spectroscopic techniques such as infrared (IR) spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, and mass spectrometry can be used to identify functional groups. These techniques provide information about the molecular structure and the presence of specific functional groups.
Understanding Reaction Mechanisms
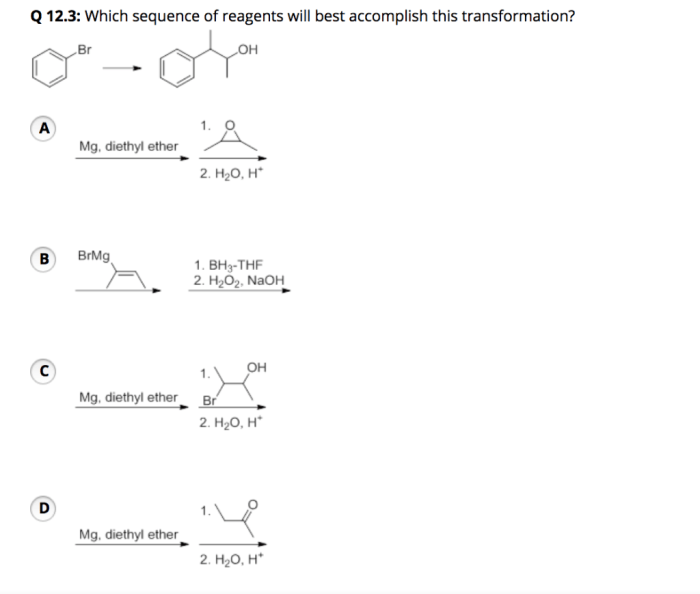
Reaction mechanisms describe the step-by-step process by which reactants are transformed into products. Understanding reaction mechanisms is essential for selecting reagents because it allows one to predict the outcome of a reaction and identify the most efficient reagents for the desired transformation.
Common reaction mechanisms include nucleophilic substitution, electrophilic addition, and elimination reactions. Nucleophilic substitution involves the replacement of a leaving group by a nucleophile. Electrophilic addition involves the addition of an electrophile to a double or triple bond. Elimination reactions involve the removal of a small molecule from a substrate.
Reagent Compatibility and Selectivity
Reagent compatibility and selectivity are important considerations when choosing reagents. Reagent compatibility refers to the ability of two or more reagents to react without interfering with each other. Reagent selectivity refers to the ability of a reagent to react with a specific functional group without reacting with other functional groups present in the molecule.
Factors to consider when choosing reagents include pH, temperature, and solvent effects. pH can affect the reactivity of certain functional groups and the solubility of reagents. Temperature can affect the rate of reaction and the selectivity of reagents. Solvent effects can influence the solubility of reagents and the polarity of the reaction medium.
Optimization of Reaction Conditions
Optimizing reaction conditions is crucial for maximizing yield and efficiency. Reaction conditions can be optimized by varying factors such as temperature, time, and catalyst use.
Temperature can affect the rate of reaction and the selectivity of reagents. Time can affect the yield of the reaction and the formation of side products. Catalyst use can accelerate the reaction rate and improve the selectivity of reagents.
Safety Considerations
Safety considerations are paramount when working with reagents. Reagents can be hazardous and can cause harm if not handled properly.
Potential hazards associated with reagents include toxicity, flammability, and corrosivity. It is important to read the safety data sheet (SDS) for each reagent and to follow proper handling and disposal procedures.
General Inquiries: Which Sequence Of Reagents Will Best Accomplish This Transformation
What factors should be considered when selecting reagents for a chemical transformation?
The selection of reagents should consider factors such as functional group compatibility, reaction mechanisms, reagent selectivity, pH, temperature, solvent effects, and safety considerations.
How can reaction mechanisms guide the selection of reagents?
Reaction mechanisms provide insights into the step-by-step transformations of reactants to products. Understanding reaction mechanisms allows chemists to identify the key intermediates and transition states involved, which in turn helps in selecting reagents that can facilitate the desired chemical changes.
What is the importance of optimizing reaction conditions for a successful transformation?
Optimizing reaction conditions involves adjusting parameters such as temperature, time, and catalyst use to maximize the yield and efficiency of a transformation. By optimizing conditions, chemists can minimize side reactions, improve product selectivity, and reduce reaction times.