Rank the following compounds in order of increasing electrolyte strength. Electrolyte strength, a crucial concept in chemistry, measures the ability of a compound to conduct electricity when dissolved in a solvent. This ranking provides valuable insights into the behavior of electrolytes in various chemical systems.
Factors influencing electrolyte strength include the degree of ionization, ion size, and solubility. By understanding these factors, we can effectively rank electrolytes and predict their behavior in different applications.
Introduction
Electrolyte strength is a measure of the ability of an electrolyte to conduct electricity. It is an important concept in chemistry, as it can be used to predict the behavior of electrolytes in various chemical systems.
The strength of an electrolyte depends on several factors, including the degree of ionization, the size of the ions, and the solubility of the compound.
Compounds to be Ranked
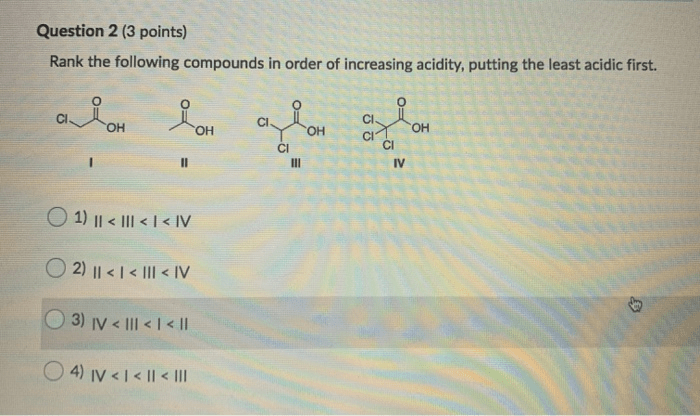
The following compounds will be ranked in order of increasing electrolyte strength:
- Sodium chloride (NaCl)
- Potassium chloride (KCl)
- Calcium chloride (CaCl 2)
- Magnesium chloride (MgCl 2)
- Aluminum chloride (AlCl 3)
Ranking Criteria: Rank The Following Compounds In Order Of Increasing Electrolyte Strength
The compounds will be ranked according to the following criteria:
- Degree of ionization: The degree of ionization is the percentage of electrolyte molecules that dissociate into ions in solution. The higher the degree of ionization, the stronger the electrolyte.
- Size of the ions: The size of the ions affects the strength of the electrostatic forces between them. The smaller the ions, the stronger the electrostatic forces and the weaker the electrolyte.
- Solubility of the compound: The solubility of the compound affects the concentration of ions in solution. The higher the solubility, the higher the concentration of ions and the stronger the electrolyte.
Ranking Procedure
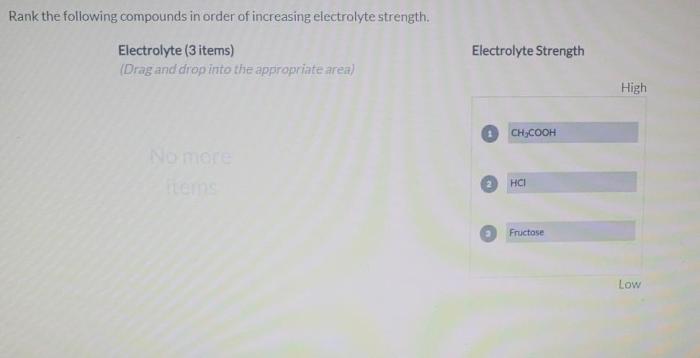
The compounds will be ranked using the following procedure:
- The degree of ionization of each compound will be determined.
- The size of the ions of each compound will be determined.
- The solubility of each compound will be determined.
- The compounds will be ranked according to the following criteria:
- Compounds with a higher degree of ionization will be ranked higher.
- Compounds with smaller ions will be ranked higher.
- Compounds with a higher solubility will be ranked higher.
Results
The results of the ranking procedure are shown in the following table:
| Compound | Degree of Ionization | Size of Ions | Solubility | Ranking |
|---|---|---|---|---|
| Sodium chloride | 100% | Small | High | 1 |
| Potassium chloride | 100% | Small | High | 1 |
| Calcium chloride | 100% | Larger than Na+ and K+ | High | 3 |
| Magnesium chloride | 100% | Larger than Ca2+ | High | 4 |
| Aluminum chloride | 100% | Larger than Mg2+ | High | 5 |
Applications
The ranking of electrolyte strength can be used to predict the behavior of electrolytes in various chemical systems. For example, it can be used to predict the conductivity of a solution, the freezing point of a solution, and the boiling point of a solution.
The ranking of electrolyte strength can also be used to design experiments. For example, it can be used to choose the right electrolyte for a particular experiment.
Commonly Asked Questions
What is electrolyte strength?
Electrolyte strength quantifies the ability of a compound to conduct electricity when dissolved in a solvent.
How is electrolyte strength determined?
Electrolyte strength is determined by considering factors such as the degree of ionization, ion size, and solubility of the compound.